Rehabtronics Announces Health Canada Approval for Prelivia Device
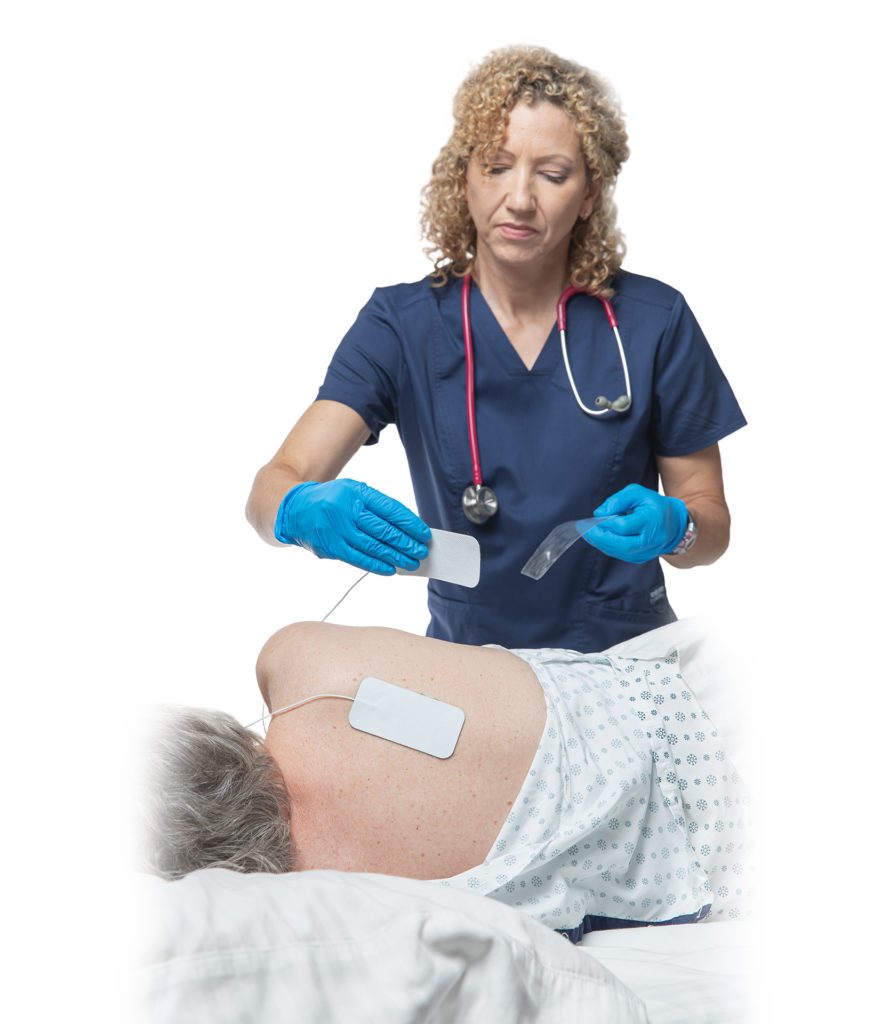
VANCOUVER, British Columbia, Aug. 22, 2024 /PRNewswire/ — Rehabtronics, a leader in advancing neurostimulation technology, is pleased to announce that Health Canada has approved the Prelivia device, an innovative device designed to address the critical issue of tissue oxygenation, the primary cause of pressure injuries in immobile patients.
Pressure injuries, also known as bedsores, impact millions of patients annually, resulting in serious complications and prolonged hospital stays. According to recent data from the Canadian Institute for Health Information, the incidence of hospital-acquired pressure injuries (HAPI) has increased by 43% over the past four years (2018-2022) and continues to rise – making HAPIs the fastest growing hospital harm event. These injuries are also a frequent medical issue for individuals with paralysis and immobility, including those living with spinal cord injuries (SCI), chronic stroke, muscular sclerosis, Parkinson’s, and the elderly, who are all at an elevated risk.
“We are thrilled to receive Health Canada’s approval for Prelivia,” said Rahul Samant, CEO of Rehabtronics. “As a Canadian business, this marks a significant milestone in our mission to improve patient outcomes and enhance the quality of care for those in Canada. With Prelivia, Canadian healthcare providers have a revolutionary new tool that enables them, for the first time, to intervene at the tissue ischemia phase of pressure injury development.”
The Prelivia device is based on Rehabtronics’ proprietary technology, which has been tested in clinical trials. It is designed for ease of use in hospital settings, offering a non-invasive and effective solution for patients at risk of pressure injuries.
“The approval of Prelivia by Health Canada validates our commitment to advancing our neurostimulation technology,” said Dr. Bojana Turic, Chief Medical Officer – VP Regulatory Affairs at Rehabtronics. “We look forward to working closely with healthcare providers across Canada to implement this device and improve the standard of care for patients nationwide.”
Rehabtronics will be rolling out the Prelivia device across Canada in the coming months, with plans to expand its availability to international markets in the near future.
For more information about Prelivia and other innovative solutions from Rehabtronics, please visit [https://rehabtronics.com].
About Rehabtronics
Rehabtronics develops medical devices that restore function and improve the lives of people who are paralyzed or immobile. Founded in 2003 as a spinoff from the Neuroscience Institute of the University of Alberta, Rehabtronics is dedicated to bringing neuroscience discoveries into clinical practice. Prelivia, the company’s newest product, is designed to alleviate pressure injuries, one of the deadliest hospital-acquired injuries. Its rehabilitation devices help people recover movement after central or peripheral nervous system injury or disease.
Media Contact:
Neha Doshi
Marketing Manager
382130@email4pr.com
+1.780.701.5167