Rehabtronics Receives Certification for Medical Device Single Audit Program (MDSAP), Expediting Global Commercialization of its Upper Limb Rehabilitation Devices
This Quality Assurance Certification confirms Rehabtronics has achieved regulatory audit and compliance requirements in numerous countries including the United States and Canada.
VANCOUVER, BC, January 3, 2024/PRNewswire/ – Rehabtronics Inc announces it has successfully upgraded its ISO13485:2016 quality certification to include the internationally recognized Medical Device Single Audit Program (MDSAP) standard. The additional certification confirms the company’s medical device manufacturing program achieves the regulatory audit requirements and compliance standards of Australia, Canada, and the United States.
“This certification is an important milestone for our company as it streamlines and expedites our efforts to seek regulatory approval to bring our rehabilitation products to new markets,” says Bojana Turic, Chief Scientific Officer-Regulatory Affairs. “By attesting that our products adhere to the highest quality standards set by regulatory bodies including the US FDA and Health Canada, MDSAP certification reduces barriers and saves us valuable time and resources.”
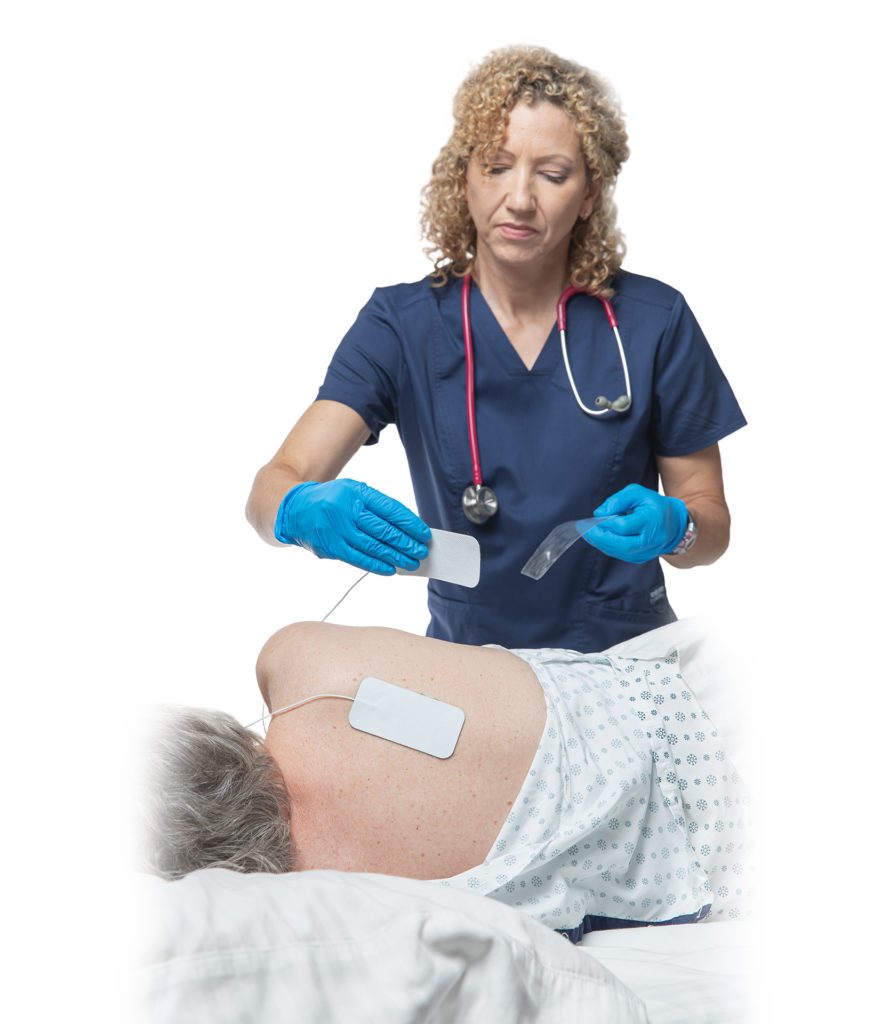
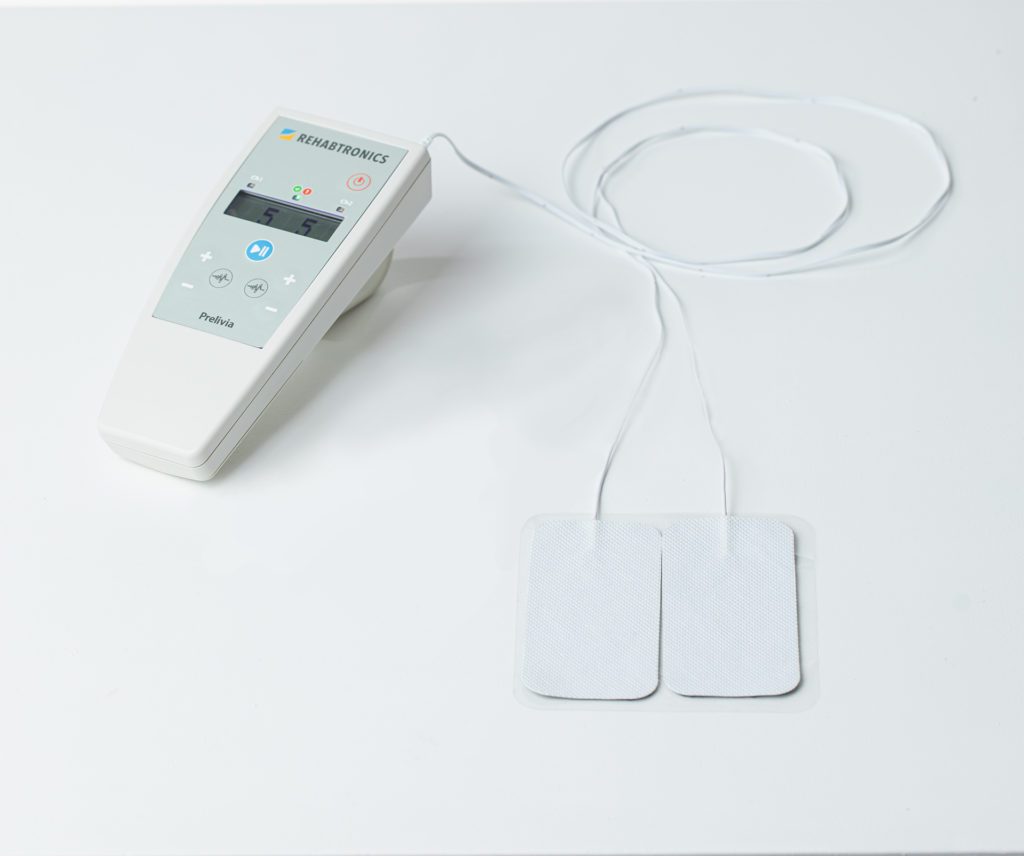
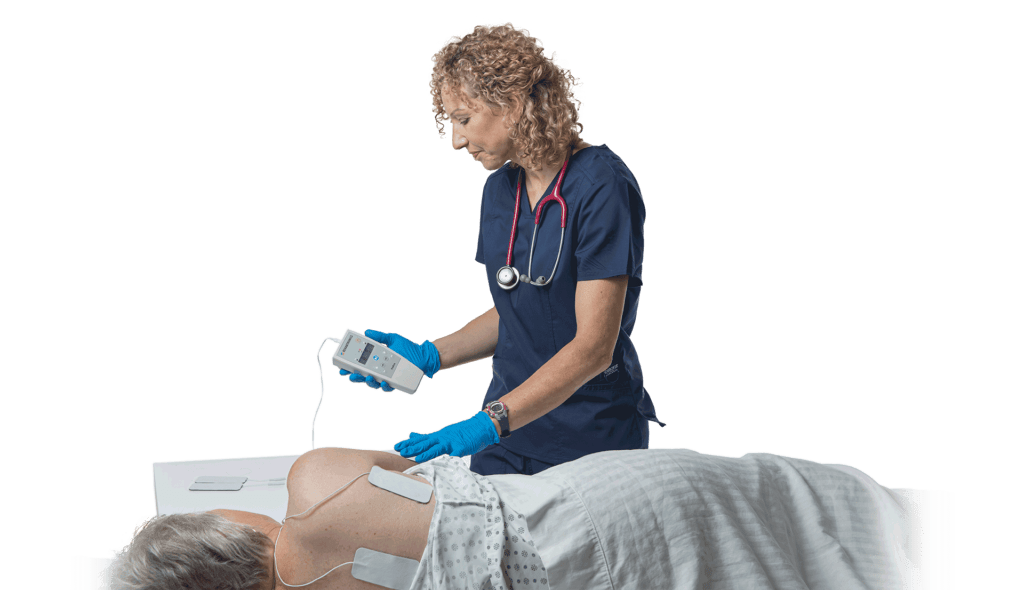
Rehabtronics currently has regulatory clearance in Europe, Asia, and the United States for its Class II neurorehabilitation products that assist patients in recovering movements after central or peripheral nervous system injury or disease. Prelivia, its newest product designed to alleviate pressure injuries, has been cleared by the US FDA and is pending Health Canada approval.
“Obtaining this certification will allow us to bring our Class II products to Canadians,” says Rehabtronics CEO Rahul Samant. “We’re excited about making our technology easily accessible to more patients beginning 2024”
ABOUT REHABTRONICS
Rehabtronics develops medical devices that restore function and improve the lives of people who are paralyzed or immobile. Founded in 2003 as a spinoff from the Neuroscience Institute of the University of Alberta, Rehabtronics is dedicated to bringing neuroscience discoveries into clinical practice. Prelivia, the company’s newest product, is designed to alleviate pressure injuries, one of the deadliest hospital-acquired injuries. Its rehabilitation devices help people recover movement after central or peripheral nervous system injury or disease. www.rehabtronics.com.
Media Contact
Neha Doshi
Marketing Manager
370419@email4pr.com
780-701-5167