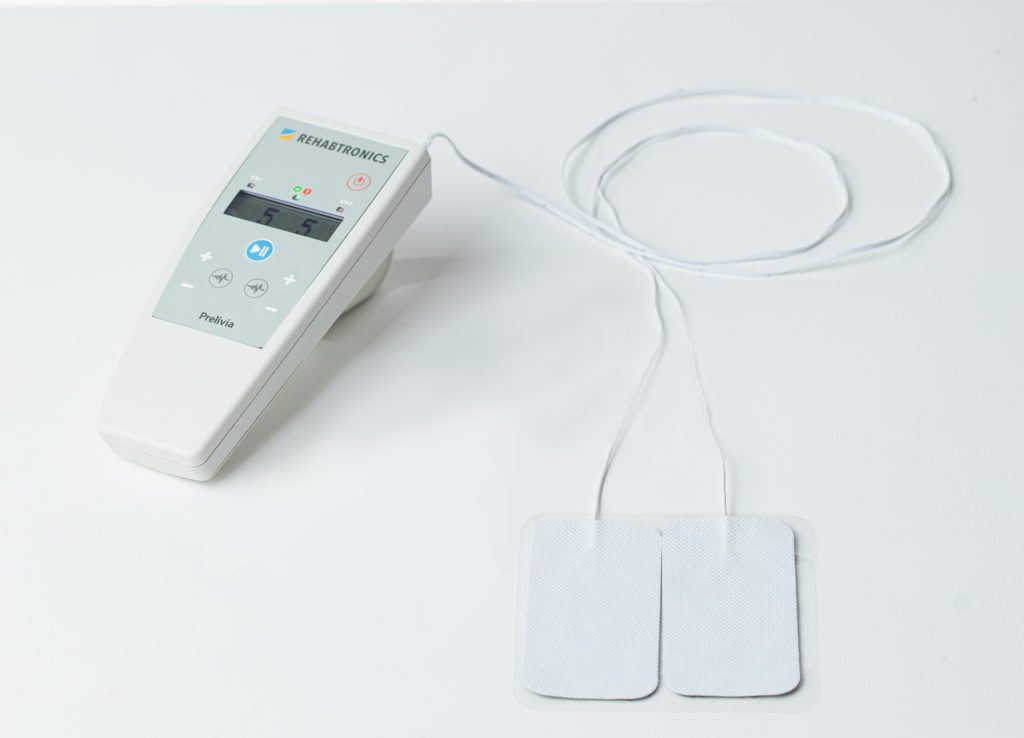
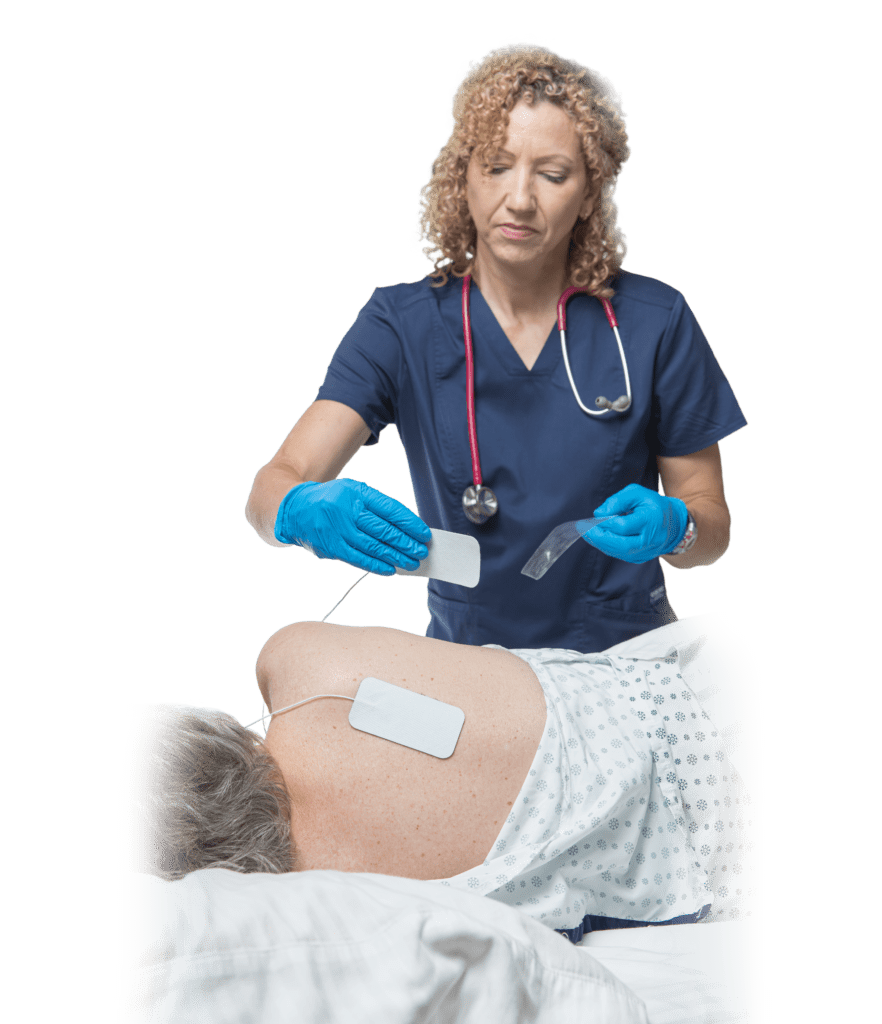
Prelivia IES1 Device
Rehabtronics has developed a proprietary neurostimulation protocol called ‘IES’. IES has been integrated into the Prelivia IES1 device. It has been established that a pressure injury may form via extrinsic and intrinsic factors. Externally, sustained pressure over bony prominences (extrinsic) and a lack of blood circulation, tissue oxygenation and tissue perfusion (intrinsic). Prelivia is the first technology to addresses both factors.
In a series of animal, human, and clinical studies it has been shown that ‘IES’ aids in:
- redistributing pressure,
- reforming compressed muscle tissue,
- increasing tissue oxygenation by 28%, and
- reduced deep tissue damage by 80%.
Clinical studies have demonstrated that Prelivia is safe and easy to deploy in hospital settings. Use by patients and health care workers demonstrates that there are no barriers to implementation and acceptance by all stakeholders.
The U.S. Food and Drug Administration has provided 510 (k) Clearance for the Prelivia neurostimulation device to be used to promote healthy blood circulation and maintains healthy tissue in people who are bedridden or chair bound.
Scroll below for details on Prelivia studies currently underway and conducted since 2010.