The Centre for Aging + Brain Health Innovation (CABHI) awarded access to its Mentorship, Capital and Continuation (MC2) program to Rehabtronics in January. The program helps companies unlock funding to accelerate business growth by providing access to leading investors, buyers, industry leaders and advisory from Silicon Valley.
Each year, the Centre for Aging and Brain Health Innovation (CABHI) awards runs its Mentorship, Capital and Continuation (MC2) program, designed to help healthtech companies whose solutions improve the lives of aging adults. Earlier this year, Rehabtronics was one of six companies to be awarded access to MC2, which unlocked access to leading investors, buyers, industry leaders and advisors from Silicon Valley. The company is already seeing results.
“Thanks to the advisors and resources from the MC2 Program, we achieved key commercialization milestones including financing, and have accelerated Prelivia’s path to market,” reports Dr. Rahul Samant, CEO of Rehabtronics.
An established company with a decade of success developing and delivering products that help people recover movement after central or peripheral nervous system injury or disease, Rehabtronics is currently seeking funding to commercialize a new product to help alleviate pressure injuries in people who are bedridden or chair bound.
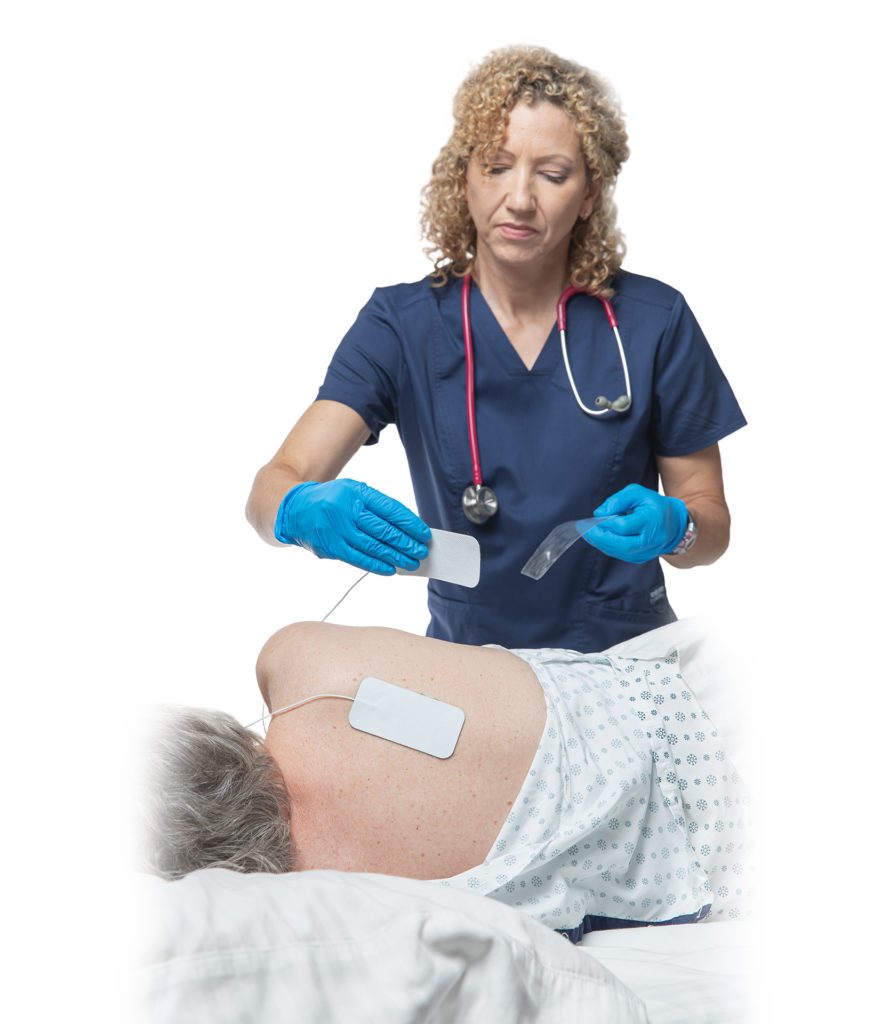
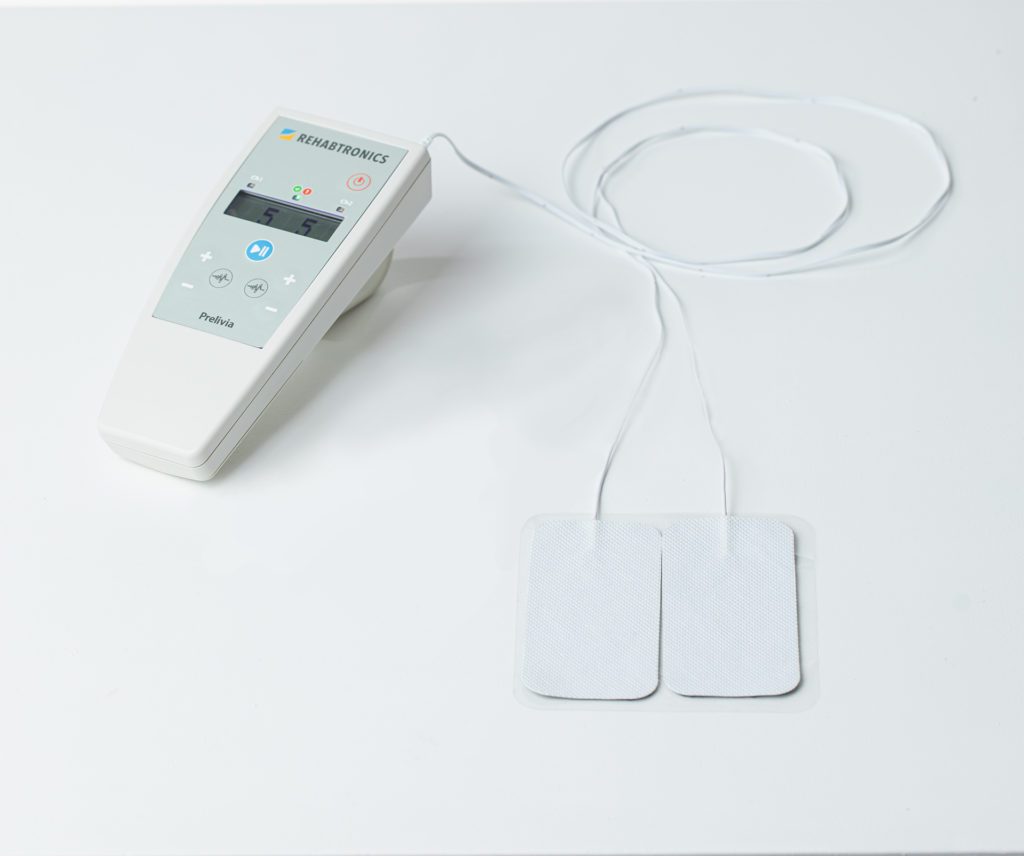
More commonly known as bedsores, pressure injuries affect up to 2.5 million patients in North America each year and are considered one of the most expensive and deadly hospital-acquired injuries. Prelivia is the first technology designed to prevent damage by restoring blood circulation and tissue oxygenation.
About Rehabtronics
Rehabtronics develops medical devices that restore function and improve the lives of people who are paralyzed or immobile. Founded in 2003, Rehabtronics is dedicated to bringing neuroscience discoveries into clinical practice. The company’s newest product, Prelivia, is designed to alleviate pressure injuries, one of the deadliest hospital-acquired injuries. Its rehabilitation devices are used globally to help people recover movement after central or peripheral nervous system injury or disease.