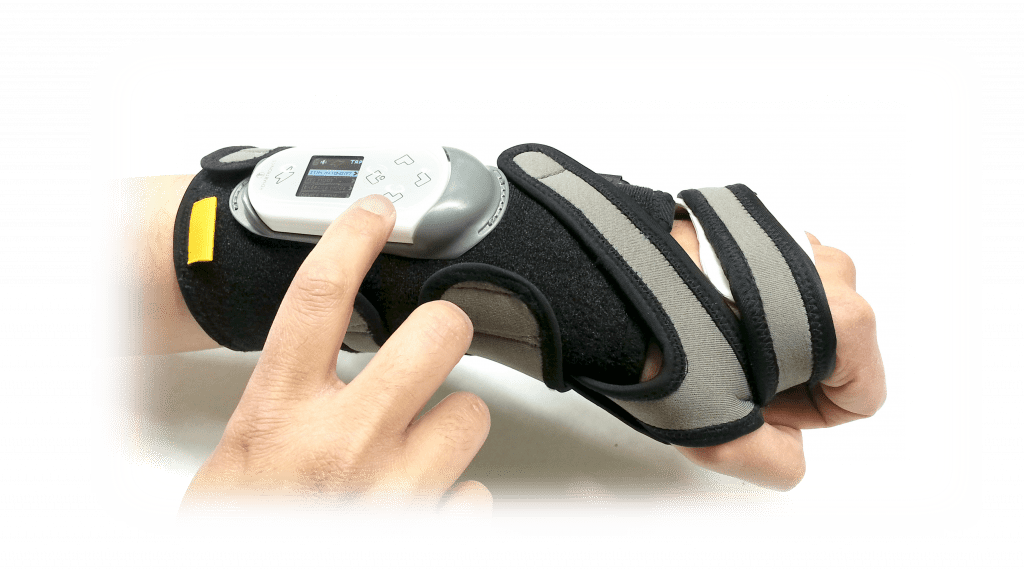
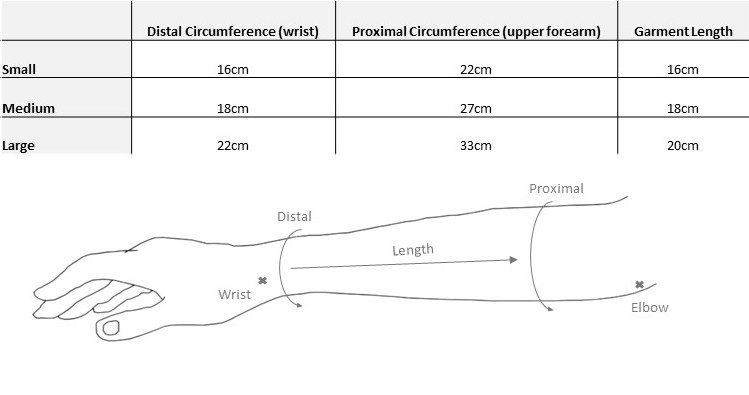
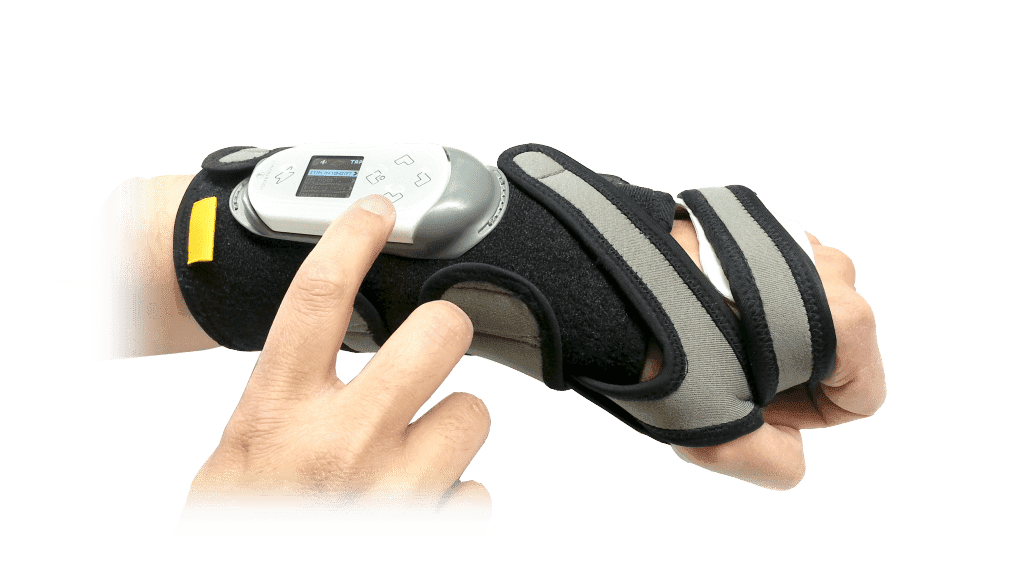
The ReGrasp has been a game-changer for me. After years of struggling with limited mobility in my right hand, this innovative device has given me newfound hope and confidence. With its comfortable design and user-friendly interface, integrating it into my routine was seamless. Within just a month and a half, I noticed significant improvements in the mobility and strength of my hand, allowing me to perform daily tasks with ease.
Functional Rehabilitation at Your Fingertips
For most stroke survivors, performing simple, daily tasks can be a frustrating struggle. Traditional rehabilitation methods are slow to deliver results and many find it difficult to recover basic capabilities.
That’s why we’ve developed the ReGrasp Hand Neurorehabilitation System. It uses functional electrical stimulation to improve hand function and range of motion.
Use the ReGrasp as a therapy tool too speed up hand rehabilitation post stroke or injury.
You can also use ReGrasp to accomplish daily tasks using head motions while wearing the discrete wireless head piece or use the buttons on the ReGrasp controller.
Our ultimate goal is to help you regain hand function, independence and improve your quality of life.