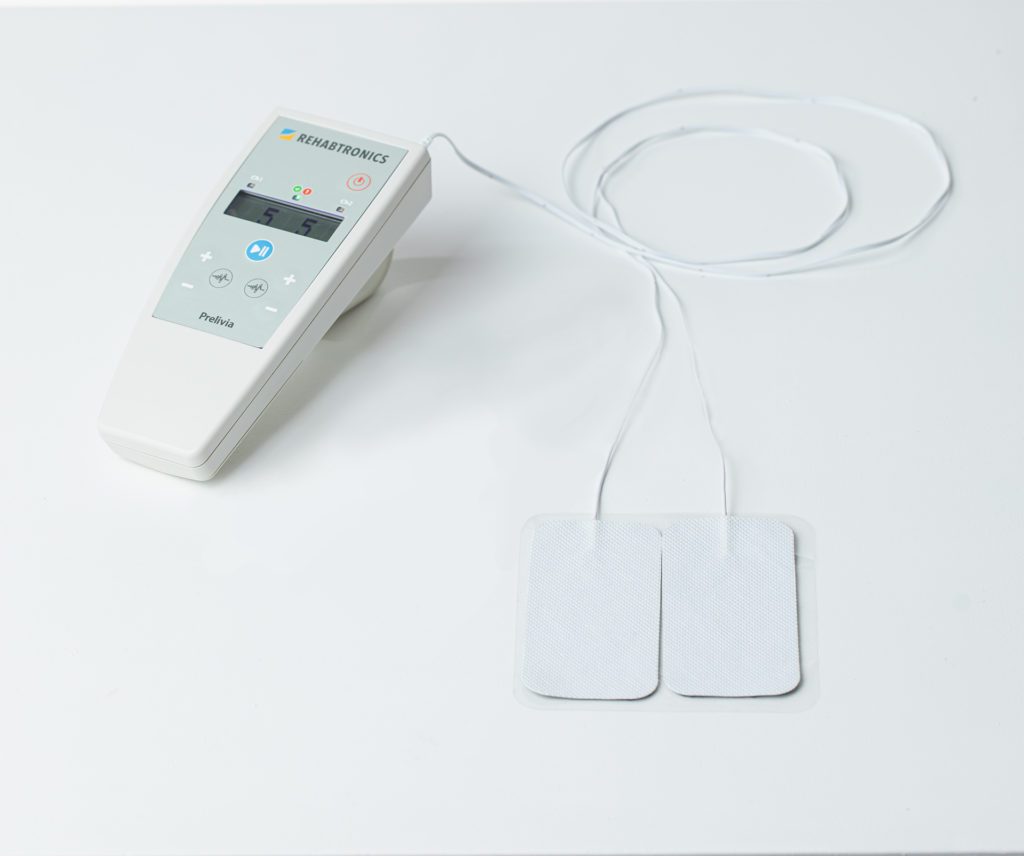
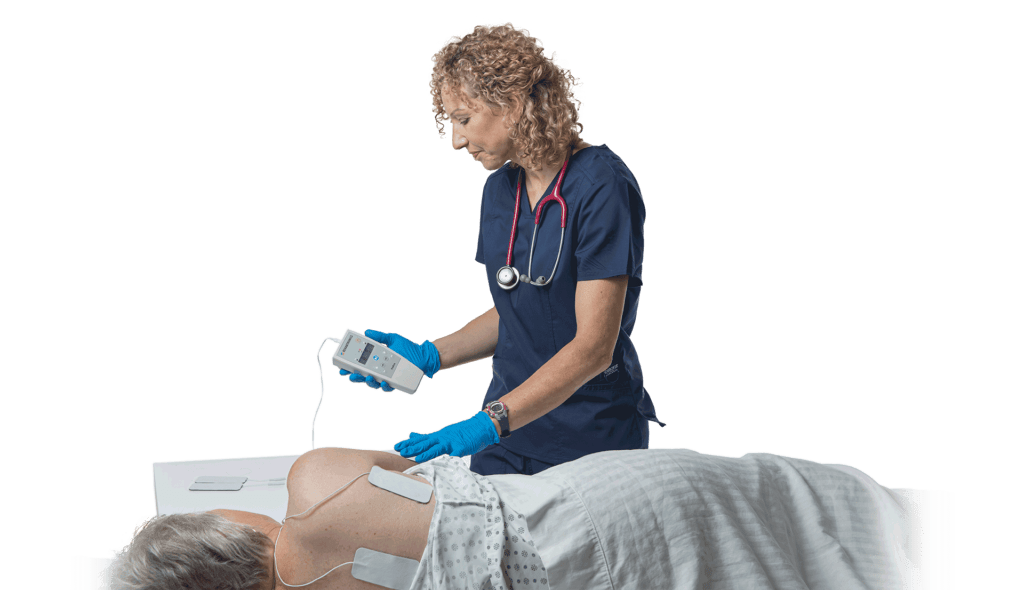
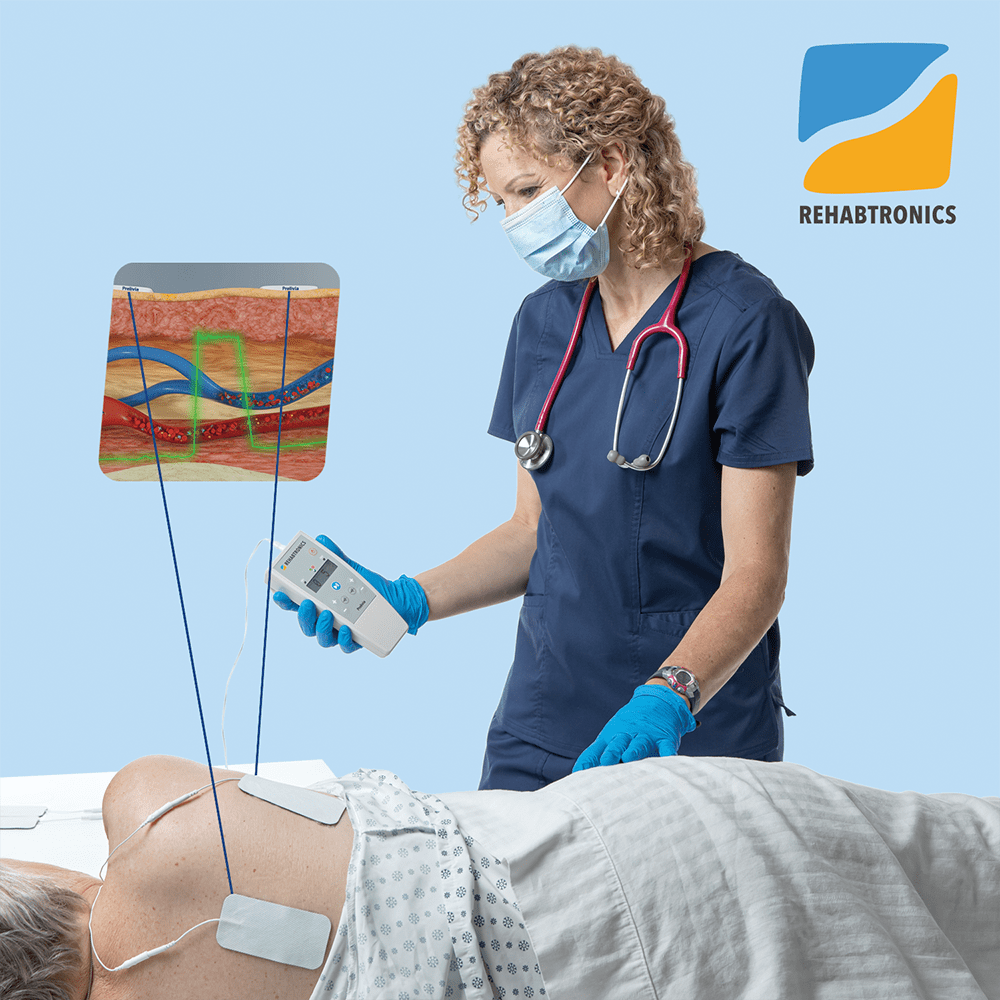
ReGrasp Bionic Glove: Gain Independence After a Stroke or Injury
ReGrasp Bionic Glove: An Innovative Way to Achieve Independence After a Stroke or Injury In preparation for introducing the ReGrasp Bionic Glove in the United States, we worked with Ashley…