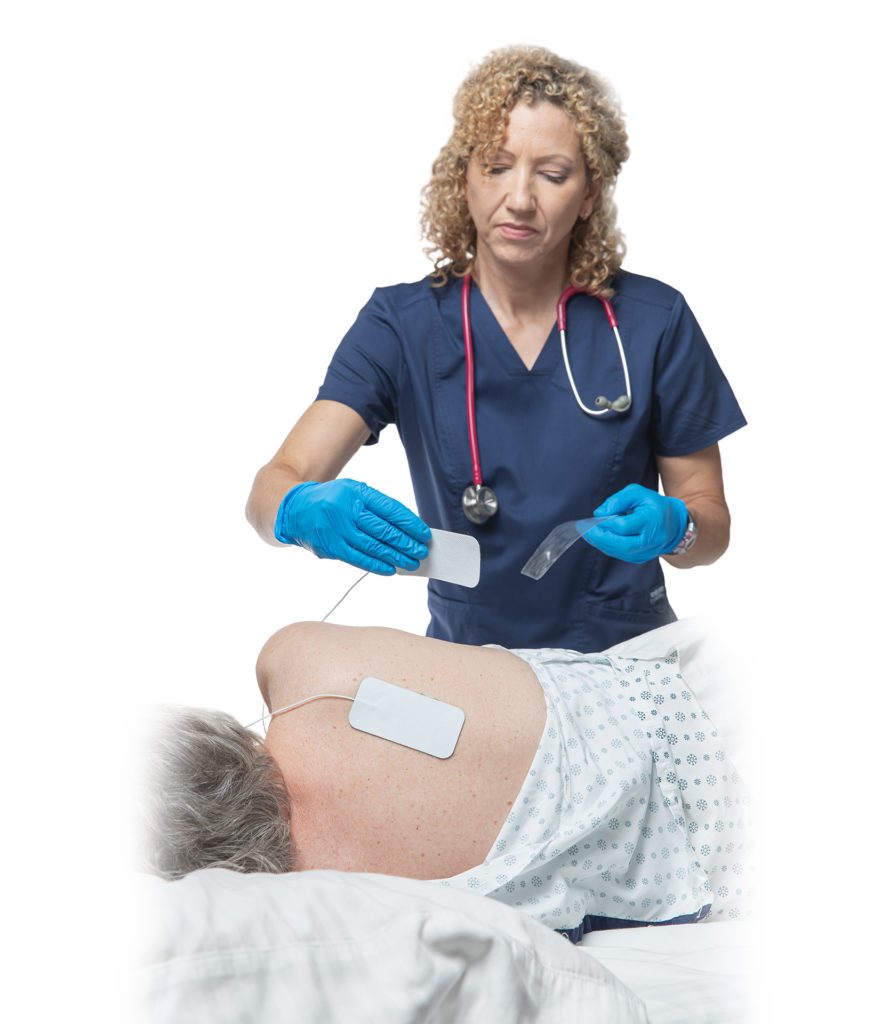
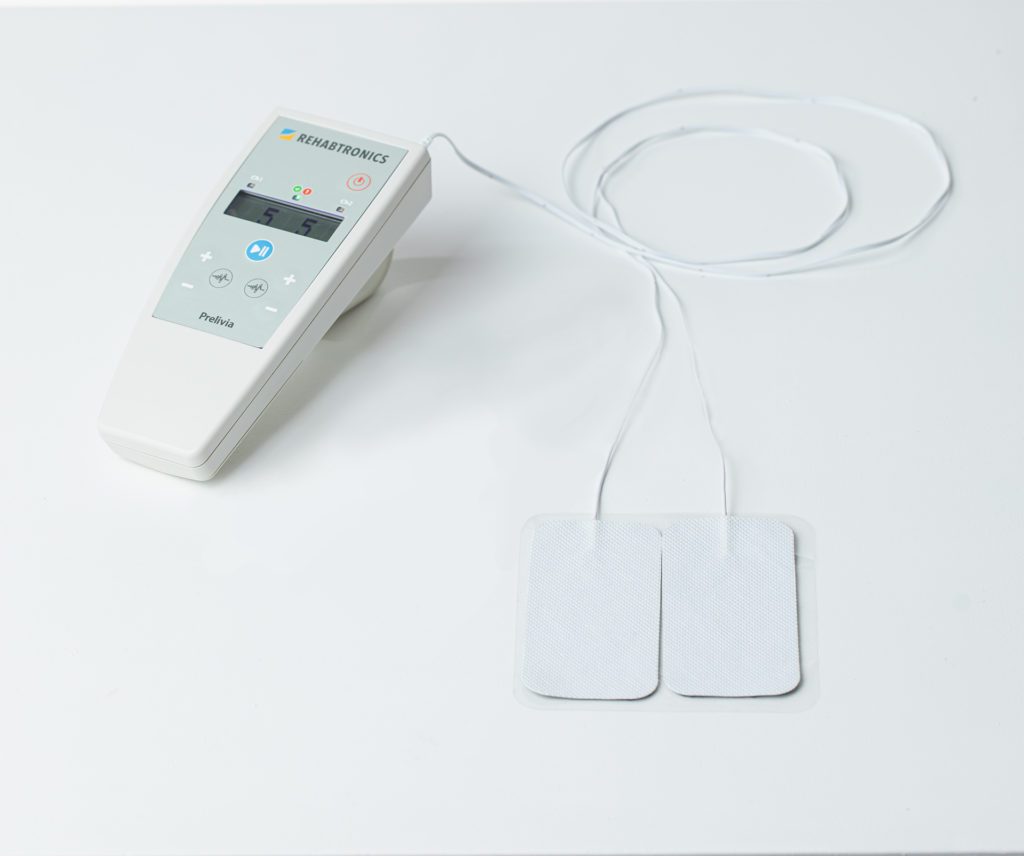
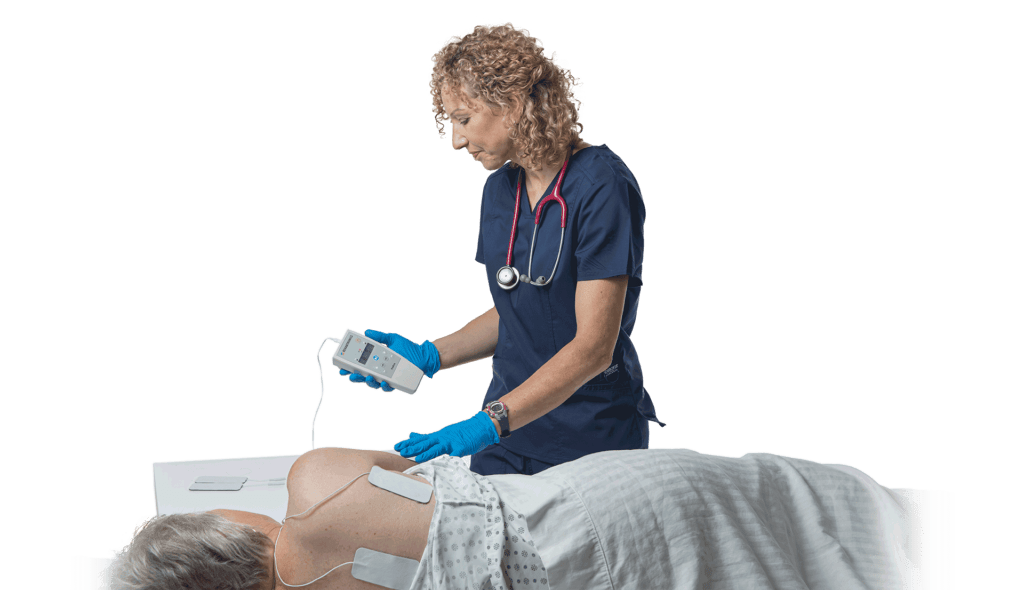
Rehabtronics to Run Prelivia Pilot Program at Providence Health Care
Rehabtronics Selected by Praxis Spinal Cord Institute to Launch Prelivia Pilot Program with Providence Health Care Ventures, Pioneering Innovative Solutions to Combat Pressure Injuries VANCOUVER, BC, Feb. 6, 2024 /PRNewswire/ —…